Recent News
Intellijoint Surgical announces second product approval in Japan; Intellijoint KNEE
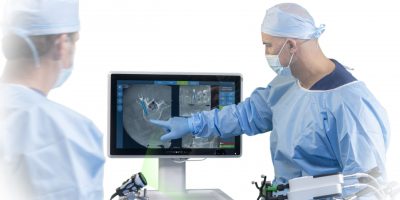
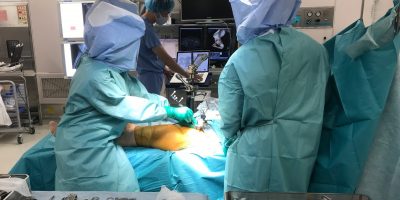
Kitchener, ON – Thursday, March 7, 2024- Intellijoint Surgical is pleased to announce regulatory approval for Intellijoint KNEE for use in Japan. This milestone marks Intellijoint’s second product for Orthopaedics in the world’s largest computer-assisted surgical navigation market. Intellijoint KNEE® is enabling technology for Orthopaedic Surgeons, providing real-time measurements of surgical cutting guides during total […]
Read Press Release